
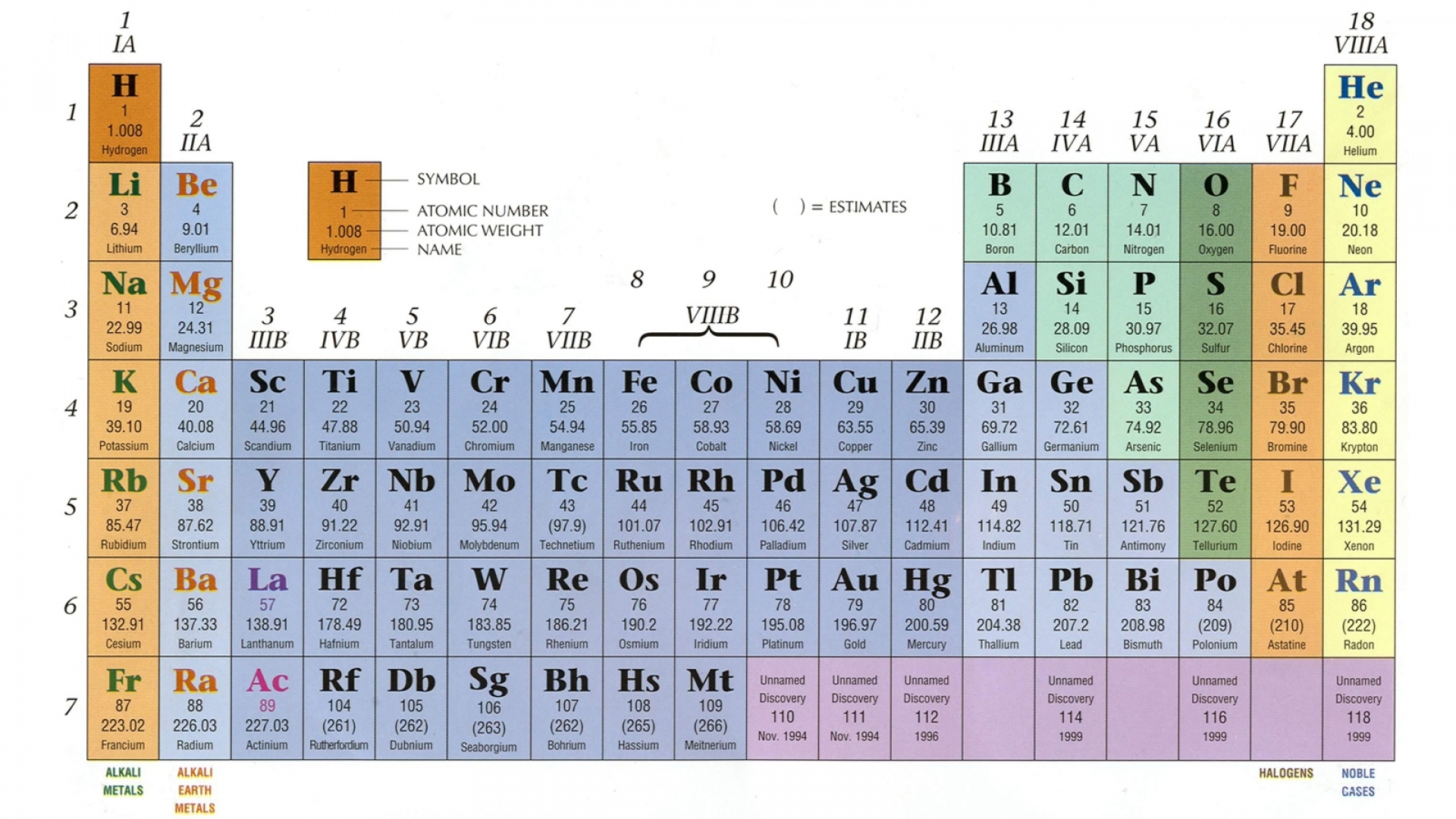
In semiconductors, electrons may reach the conduction band, when they are excited, for example, by ionizing radiation (i.e. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. In electrical insulators and semiconductors, the conduction band is the lowest range of vacant electronic states. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
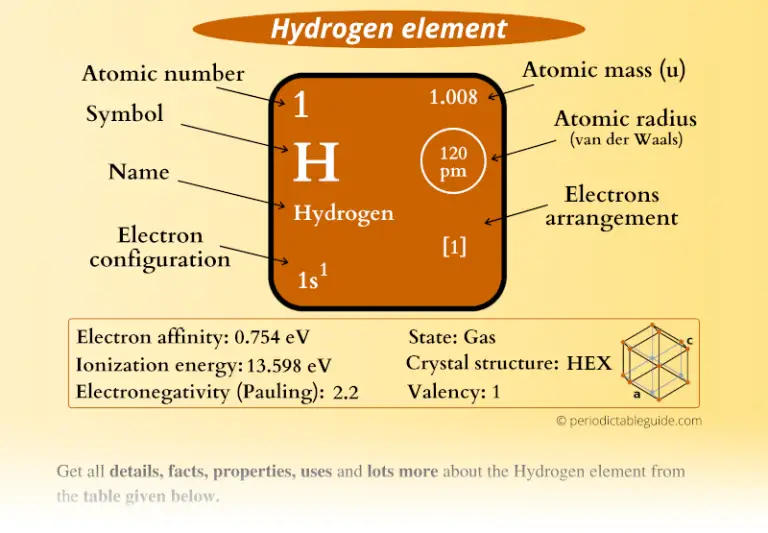
Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. In the ground state, they are arranged in the electron configuration 3s 23p 2. For example, a silicon atom has fourteen electrons. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. To understand the difference between metals, semiconductors and electrical insulators, we have to define the following terms from solid-state physics: from ionizing radiation) to cross the band gap and to reach the conduction band. In contrast to conductors, electrons in a semiconductor must obtain energy (e.g. In solid-state physics, this energy gap or band gap is an energy range between valence band and conduction band where electron states are forbidden. They have an energy gap less than 4eV (about 1eV). The name semiconductor comes from the fact that these materials have an electrical conductivity between that of a metal, like copper, gold, etc. Semiconductors are materials, inorganic or organic, which have the ability to control their conduction depending on chemical structure, temperature, illumination, and presence of dopants. Insulators, on the other hand, are made of a wide variety of materials depending on factors such as the desired resistance. Conductors are made of high-conductivity materials such as metals, in particular copper and aluminium. Substances in which electricity can flow are called conductors. While resistivity is a material property, resistance is the property of an object. Electrical resistance is expressed in Ohms. Note that, electrical resistivity is not the same as electrical resistance. The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). The symbol of resistivity is usually the Greek letter ρ (rho). A low resistivity indicates a material that readily allows the flow of electric current. m.Įlectrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current.Hydrogen – Electrical Resistivity and Electrical ConductivityĮlectrical resistivity of Hydrogen is - nΩ


 0 kommentar(er)
0 kommentar(er)
